Abstract
Introduction
Adoptive immunotherapy with CD4+CD25+FOXP3+ regulatory T cells (Tregs) and conventional T cells (Tcons) has been employed after allogeneic hematopoietic stem cell transplantation (HSCT) to prevent Graft-versus-Host Disease (GvHD) while mantaining Graft-versus-Leukemia effect in the absence of any further immune suppressive GvHD prophylaxis (Di Ianni, Blood 2011; Martelli, Blood 2014). A recent study of age-adapted haploidentical HSCT with Treg/Tcon adoptive immunotherapy (Treg/Tcon haplo-HSCT) resulted in an unprecedented 75% chronic GvHD (cGvHD)/leukemia-free survival (CRFS) in acute myeloid leukemia patients. Acute GvHD (aGvHD) occured in 33% of patients and was the most frequent complication of the procedure (Pierini, Blood Advances 2021). Here, we analyzed clinical and histological characteristics of aGvHD and evaluated its impact on outcomes in patients who received Treg/Tcon haplo-HSCT.
Methods
We retrieved data of patients who underwent Treg/Tcon haplo-HSCT at Perugia University Hospital and who were evaluable for aGvHD from January 2015 until June 2021 (clinicaltrials.gov: NCT03977103). Histological features and lymphoid infiltration by immunohistochemistry were analyzed in gut samples of patients who received diagnostic colonscopy and biopsy for gastrointestinal symptoms.
Results
A total of 105 patients engrafted after Treg/Tcon haplo-HSCT. Mean age at transplant was 48 years (range 18-70). Acute leukemia was the most frequent diagnosis (78/105 myeloid, 23/105 lymphoblastic), 4 patients had myelodisplastic syndrome and 2 multiple myeloma. Twenty-seven (26%) patients had active disease at the time of HSCT. Fourteen (13%) patients received Treg/Tcon haplo-HSCT as a second HSCT procedure. Thirty-one (29%) patients developed grade II-IV aGvHD. Four patients had grade II aGvHD, 23 grade III, and 4 grade IV. Almost all patients (90%) presented gut involvement, while skin was involved in 64% of them and liver in 35%. To explain why gut symptoms such as diarrhea and abdominal pain were more frequently present than symptoms of skin or liver involvement, we evaluated 40 histological samples from 34 patients who received colonoscopy for suspected aGvHD. Twenty-two of them (65%) received a clinical diagnosis of aGvHD and pharmacological immune suppression was promptly started. Clinical diagnosis did not always match histological findings: biopsies were suggestive of aGvHD in 20/22 patients who required treatment, but also in 8/12 patients who did not. We found no difference in median CD3+ cell infiltration among patients who did not need immune suppressive treatments and patients with aGvHD (460 cells/mmq vs 446 cells/mmq). However, FOXP3+ Tregs preferentially infiltrated gut mucosa of patients who resolved clinical symptoms with no need of immune suppressive treatments (120 cells/mmq vs 59 cells/mmq, p = 0.008; FOXP3/CD3 ratio: 0.27 vs 0.18, p = 0.004). This observation suggested Treg defective homing to gut mucosa of aGvHD patients. Indeed, we found infused Tregs expressed lower levels of gut homing receptor α4β7 in comparison with Tcons (p< .001).
Despite severe presentation at diagnosis, aGvHD resolved in the majority of patients (71%), and immune suppressive therapy could be completely withdrawn after a relatively short-course (median: 98 days, range 48-404). Sixteen/31 patients (52%) were steroid refractory and required further treatments after first-line (e.g. Ruxolitinib). Only two of them developed moderate/severe cGvHD and one is alive and off-therapy.
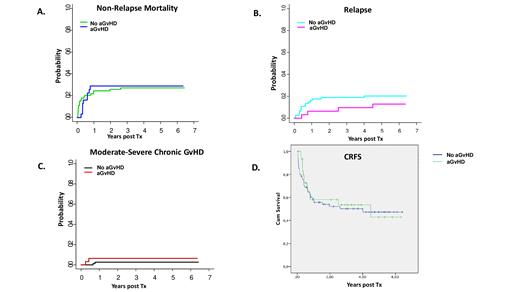
Indeed, aGvHD diagnosis did not result in higher incidence of non-relapse mortality (cumulative incidence of 29% in aGvHD patients vs 27% in controls; p=non significant, ns, Fig.1A), relapse (13% vs 20%; p=ns, Fig.1B), or cGvHD (6.5% vs 3%; p=ns, Fig.1C). In fact, CRFS of aGvHD patients was similar to CRFS of patients that did not develop aGvHD (Fig. 1D, p=ns, median follow up of live patients: 1246 days).
Conclusions
Treg/Tcon haplo-HSCT is followed by low rates of relapse and cGvHD and results in good CRFS in high risk leukemia patients. aGvHD after Treg/Tcon haplo-HSCT preferentially involves the gut where Treg homing is defective. Strategies that enhance Treg homing in the gut (e.g. low-dose IL-2) may help reduce aGvHD after Treg/Tcon haplo-HSCT. In conclusion, despite aGvHD occurred in 29% of patients, it was responsive to treatments and it did not result in disease relapse or cGvHD.
No relevant conflicts of interest to declare.